DTG: Professional medical device injection molding solution provider
1. Customized design and development
We work with our customers to understand their needs and product requirements and provide customized design and development solutions. Whether it is a simple part or a complex medical device assembly, we are able to provide professional technical support and innovative design solutions.
2. Material selection and process optimization
We have extensive experience in material selection and process optimization. We work with a variety of material suppliers to ensure that we provide our customers with materials that meet industry standards and regulatory requirements. Through sophisticated process optimization, we are able to achieve high accuracy and stability of medical device injection molded parts.
3. Quality Control and Certification
At DTG, we consider quality as life. We employ strict quality control measures to ensure that every medical device injection molded product meets the highest standards. Our production process complies with ISO 13485 quality management system, as well as relevant standards such as FDA and EU CE certification.
4. Fast delivery and flexible service
We understand the importance of time in the medical device industry. Therefore, we are committed to providing fast delivery and flexible services. Whether it is a small batch or large scale production, we are able to meet the needs of our customers and ensure timely delivery.
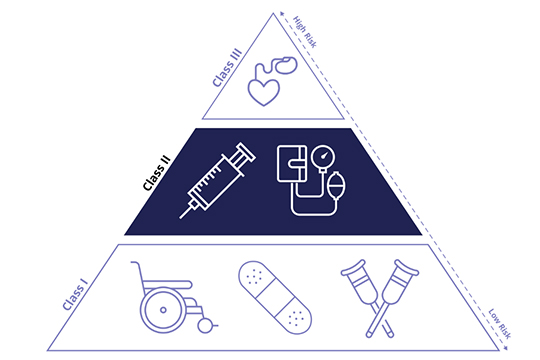
Medical Product Classification
1.Class i Medical Products
Class I medical devices are considered low-risk and are subject to the least regulatory controls. Include:Tongue depressors、Bandages and adhesive tapes、Surgical gloves、Non-powered wheelchairs、Examination gloves.
2.Class ii Medical Products
These devices are considered to pose moderate risk to the patient or user, requiring stricter regulatory controls than Class I devices. products include:Infusion pumps、Blood pressure monitors、Powered wheelchairs、X-ray machines.
3.Class iii Medical Products
Commonly used to support or sustain human life and important in preventing impairment of human health. Products include: Implantable pacemakers & defibrillators、Artificial hearts、Intracranial stents、Cochlear implants.
Common medical device injection molding Applications
Common Medical Device Injection Molding Applications include the production of surgical instruments, drug delivery devices, diagnostic equipment components, and disposable medical supplies.
Why Choose DTG Injection Molding Services ?

ISO Certified
ISO Certified
Expertise
Advanced Equipment
Fast delivery
Customer Focus
Our Mold Design Process
For simple parts, product lead times can be reduced to as little as 3 days.Upload your part to see if it qualifies.
01
Requirement Analysis
We start by thoroughly understanding the project requirements, including the part geometry, material properties, production volume, and budget constraints. This initial phase involves close collaboration with the client to gather all necessary information and establish clear design objectives.
02
Conceptual Design
Our team of skilled designers and engineers utilizes advanced CAD software to create conceptual designs based on the project requirements. This phase involves brainstorming innovative solutions and exploring various design options to achieve the desired functionality and manufacturability.
03
Detailed Design
Once the conceptual design is approved, we proceed to the detailed design phase. This stage involves refining the 3D model to incorporate all essential features, such as parting lines, gating systems, cooling channels, and ejection mechanisms. Our designers pay meticulous attention to detail to optimize the mold design for efficiency and performance.
04
Simulation & Analysis
Once the conceptual design is approved, we proceed to the detailed design phase. This stage involves refining the 3D model to incorporate all essential features, such as parting lines, gating systems, cooling channels, and ejection mechanisms. Our designers pay meticulous attention to detail to optimize the mold design for efficiency and performance.
05
Design Review & Optimization
The design undergoes thorough review by our team of experts, where potential issues or areas for improvement are identified and addressed. We collaborate closely with the client to incorporate feedback and make any necessary adjustments to optimize the mold design for manufacturability and functionality.
06
Prototype Development
Upon finalizing the design, we proceed to prototype development to validate the mold’s functionality and performance. This stage involves manufacturing prototype molds and conducting rigorous testing to ensure they meet the client’s requirements and quality standards.
07
Finalization & Production Release
Once the prototypes are successfully tested and approved by the client, we finalize the mold design and release it for full-scale production. Our team closely monitors the manufacturing process to ensure the molds are fabricated to the highest standards and deliver consistent, high-quality parts.
08
Continuous Improvement
At Omni, we are committed to continuous improvement and innovation. We regularly evaluate our mold design process to incorporate the latest technologies, best practices, and customer feedback, ensuring we deliver exceptional results with every project.